40 fda approved drug labels
FDA Drug Labeling Product Requirements, Guidance - PDG FDA's Guidance for Industry entitled "Help-Seeking" and Other Disease Awareness Communications by or on Behalf of Drug and Device Firms (January 2004) describes two types of drug labeling: FDA-approved labeling, and promotional labeling. An example of FDA-approved labeling is the Professional Package Insert (PPI). Table of Pharmacogenomic Biomarkers in Drug Labeling | FDA The table below lists therapeutic products from Drugs@FDA with pharmacogenomic information found in the drug labeling. The labeling for some, but not all, of the products includes specific actions...
Drug Labels | FDA Drug Labels This is a partial collection of labeling submitted to the FDA Center for Veterinary Medicine (FDA CVM) by animal drug sponsors for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and...

Fda approved drug labels
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/17/2021: ORIG-1: Approval Label (PDF) Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 04/28/2022: ORIG-1: Approval Label (PDF) Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) As of November 10, 2020, PharmGKB listed a total of five drug approvals with "recommended testing", of which two (i.e., azathioprine and thioguanine) were first approved prior to 2000 and to which biomarker information was added as part of subsequent labeling updates [ 62 ].
Fda approved drug labels. Changes in Companion Diagnostic Labelling: Implementation of FDA's ... Advanced understanding of the molecular pathways of oncologic diseases has shifted therapeutic treatment development to focus on mechanism of actions targeting specific genomic alterations. These precision medicines are indicated for patient subsets defined by these specific mutations as determined by diagnostic devices approved by the Food and Drug Administration (FDA). The Intended Use ... Animal Drugs @ FDA 10903 New Hampshire Avenue Silver Spring, MD 20993 1-888-INFO-FDA (1-888-463-6332) Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ... Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 03/23/2022: ORIG-1: Approval Label (PDF)
Full Issue of Drug Industry Daily | 2022-06-15 | FDAnews Drug Industry Daily (ISSN 1541-6607), an executive briefing of developments at the FDA and in the pharmaceutical industry, is published daily, 250 issues, for $1,695. Photocopying or reproducing in any form, including electronic or facsimile transmission, scanning or electronic storage is a violation of federal copyright law and is strictly ... Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 02/11/2021: ORIG-1: Approval Label (PDF) Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 12/21/2020: SUPPL-34: Efficacy-Labeling Change With Clinical Data DailyMed Sep 15, 2021 · The DailyMed RSS feed provides updates and information about new drug labels approved by the FDA and published on NLM's DailyMed Web site. About DailyMed The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use ...
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web... A first-of-its-kind alopecia treatment that triggers hair growth was ... Alex Papp/Getty Images The FDA approved the first systemic, or full-body, drug to treat the hair-loss condition alopecia. It interferes with the unwanted immune response, and had already been... FDA Approves Label Extension for Evrysdi for Infants with Spinal ... SOUTH PLAINFIELD, N.J., May 31, 2022 /PRNewswire/ -- PTC Therapeutics, Inc. (NASDAQ: PTCT) today announced that the U.S. Food and Drug Administration (FDA) has approved a label extension for Evrysdi ( risdiplam) to include infants under 2 months old with spinal muscular atrophy (SMA). "The label extension for Evrysdi to include pre-symptomatic ... New Drugs - List of Latest FDA Approvals 2022 - Drugs.com Company: Dermavant Sciences. Date of Approval: May 23, 2022. Treatment for: Plaque Psoriasis. Vtama (tapinarof) is a topical aryl hydrocarbon receptor (AhR) modulating agent indicated for the treatment of plaque psoriasis in adults. FDA Approves Vtama (tapinarof) Cream for the Treatment of Plaque Psoriasis in Adults - May 24, 2022.
FDA Label Search The drug labeling on this Web site may not be the labeling on currently distributed products or identical to the labeling that is approved. Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies described in monographs.
Drugs@FDA: FDA-Approved Drugs Action Date Submission Supplement Categories or Approval Type Letters, Reviews, Labels, Patient Package Insert Note Url; 08/13/2021: SUPPL-93: Labeling-Package Insert
FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
OTC Drug Facts Label | FDA In the Federal Register of March 1999, the Food and Drug Administration published the OTC Drug Facts Label regulation. This regulation required most OTC drug products to comply with the new format...
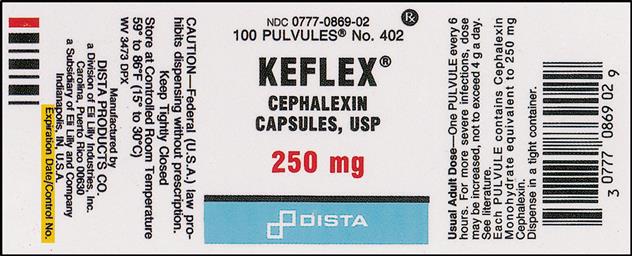
Drugs@FDA: What's in a Drug Product Label? | FDA adverse events (side effect) drug abuse and dependence. dosage and administration. use in pregnancy, use in nursing mothers. use in children and older patients. how the drug is supplied. safety ...
Drugs@FDA: FDA-Approved Drugs For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),...
FDA needs to allow generic drug manufacturers to post warning labels on prescription drugs, says ...
Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000-2020) Drug labels containing PGx information were obtained from Drugs@FDA and guidelines from PharmGKB were used to compare the actionability of PGx information in drug labels across therapeutic areas. The annual proportion of new drug approvals with PGx labeling has increased by nearly threefold from 10.3% (n = 3) in 2000 to 28.2% (n = 11) in 2020 ...
Drugs@FDA: FDA-Approved Drugs - Food and Drug Administration * Drugs@FDA includes information about drugs, including biological products, approved for human use in the United States (see FAQ), but does not include information about FDA-approved products regulated by the Center for Biologics Evaluation and Research (for example, vaccines, allergenic products, blood and blood products, plasma derivatives, cellular and gene therapy products).
FDALabel: Full-Text Search of Drug Product Labeling | FDA The FDALabel Database is a web-based application used to perform customizable searches of over 140,000 human prescription, biological, over-the-counter (OTC), and animal drug labeling documents....
Search FDA Drug Labels With WIZMED | Orange Book Search Data Upon FDA drug approval, details on FDA labels are sent to multiple agencies who publish different information. Not to mention, all the competitive intelligence drug information you are seeking is redacted and located in different databases. One Search is on a mission to bring all that data to your fingertips in just one search.
Eli Lilly wins FDA label expansion for Olumiant in hair-loss disorder The U.S. Food and Drug Administration (FDA) announced on Monday that the Eli Lilly ( NYSE: LLY) received approval for Olumiant oral tablets as a treatment for adults with severe alopecia areata, a ...
Is It Really 'FDA Approved'? | FDA May 10, 2022 · The FDA does not approve individual food labels before food products can be marketed. But FDA regulations require specific labeling elements, including nutrition information, to appear on most ...
Approved Animal Drug Products (Green Book) | FDA Feb 14, 2022 · Most FDA-approved animal drugs are included in a publicly available list of approved animal drug products. This list is called the Green Book for short, and FDA updates it in its entirety every month.
PDF HIGHLIGHTS OF PRESCRIBING INFORMATION DOSAGE FORMS ... - accessdata.fda.gov FDA-approved patient labeling. Revised:06/2022 . FULL PRESCRIBING INFORMATION: CONTENTS* 8.4 . 1 INDICATIONS AND USAGE . ... This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: June 20 22. Reference ID: 4997677. Title: label Author: fda/cder Subject: Rubraca \(rucaparib\) Tablets ...
FDA Approves Amvuttra (vutrisiran) for the Treatment of the ... The FDA approval of Amvuttra is based on positive 9-month results from HELIOS-A, a global, randomized, open-label, multicenter, Phase 3 study that evaluated the efficacy and safety of Amvuttra across a diverse group of patients with hATTR amyloidosis with polyneuropathy. 164 patients with hATTR amyloidosis were randomized 3:1 to receive either ...
Drug Labeling Overview - Food and Drug Administration The openFDA drug product labels API returns data from these submissions for both prescription and over-the-counter (OTC) drugs. The labels are broken into sections, such as indications for use...








.png)



Post a Comment for "40 fda approved drug labels"